Kinetic Hydrate Inhibitors - A History Lesson
One Person’s Journey through the development , testing and commercialization of the early KHI’s
The story for me starts 30 years ago when I worked at a chemical service company called TR Oil Services (TROS) now known as Clariant. At the time I was a Development Chemist tasked with formulating and testing new corrosion inhibitor products. I thought I had found my niche as I was able to formulate and test very effective corrosion inhibitors. In fact, in the short period of time I had been doing it, I created and commercialized a number of new corrosion inhibitors that were successfully applied in the field. So I thought I would be doing that for a few more years but unbeknownst to me I had been chosen by my boss to take over the running of the new hydrate test cell the company had just bought from BP (See picture below) to test Kinetic Hydrate Inhibitors (KHI). The main problem there was I that I knew nothing about Hydrates and KHIs so was wondering how this was all going to work.
Thankfully for me, we had a good starting point as TROS had partnered up with BP who had been developing KHI technology for a number of years before in a Joint Venture with Shell, then latterly by themselves. At the beginning the research was driven by the realization that there are Arctic fish that possess antifreeze proteins (AFPs) that prevent their blood and body fluids from freezing in extremely cold waters. These proteins, produced primarily in the liver, bind to and inhibit the growth of ice crystals, allowing fish to survive in icy habitats where temperatures can drop below the freezing point of their blood. Therefore taking that piece of information the research looked at chemical structures that could do the same job on hydrates.
As with most oil and gas research it was driven by the CAPEX and OPEX costs associated with the use of the thermodynamic inhibitors, Methanol and MEG and to prevent seeing pictures like the one below.
With their low dose rates <2% of the water volume being produced and low toxicity they were seen as an acceptable option for use offshore in the UK and Dutch Sectors of the North Sea. The exception was in Norway where there was a concern regarding the biodegradability of the KHI polymers. These polymers had <20% biodegradability, which ruled them out in Norway, even though the KHI products had very low aquatic toxicity and did not bioaccumulate. Their biodegradability was nowhere near the desired >60% biodegradability the Norwegian regulatory authority was looking for.
As part of BP’s research, they were able to formulate a KHI product that was field trialed successfully offshore in the UK Southern North Sea in 1994. (See SPE 30696 Trials of Threshold Hydrate Inhibitors in the Ravenspurn to Cleeton Line) BP initially called their KHI product a Threshold Hydrate Inhibitor (THI) but overtime this nomenclature was dropped and replaced with KHI. The problem was that THI was and is still used to describe Thermodynamic Inhibitors like MEG and Methanol.
At the time KHI were really new, only a few people that I was aware of were performing research into them. There was the joint project with Shell and BP, Exxon were very active and Rogalands Research in Norway had just started a Joint Industry Project (JIP). It was the JIP that really got me up to speed with what KHI’s were all about after TROS joined the JIP and I became the TROS rep to the JIP.
As JIP’s go I thought it was very well run and was able to identify some effective KHI polymers but unfortunately unknown to the JIP, the best polymers identified had already been patented for use as KHI’s by Exxon. So even with all the JIP’s hard work they didn’t have an end product to show for it but for me it was great experience and I got to go to lovely towns like Stavanger, Bergen and Trondheim for the first time, which was nice.
How do KHI’s work?
So now that I have given you an idea of how I got into testing, developing and commercializing KHI’s I suppose I better add some technical stuff to the post and give you an idea of how KHI’s work? They work by absorbing on the hydrate crystal via their amide functional group as the hydrate crystal starts to nucleate. A good description of them that I found was that they are the opposite of a catalyst in that they increase the amount of kinetic energy required to form hydrates not decrease the energy.
It is also worth mentioning that this is similar to the way scale and wax inhibitors work as both are kinetic inhibitors in their own way.
Taking into account how KHI’s work, this probably helps explain why KHI’s only work up to a certain degree of subcooling and then for only a certain period of time. This is because the energy that is available in some of the colder, higher pressure systems to form hydrates is just too high to adequately inhibit. So, no amount of KHI is going to stop hydrates from forming in these systems.
Currently, if you want protection for >24 hours and your system’s subcooling is >12oC subcooling then you are probably out of luck. In fact, even at subcoolings of 10-11oC subcooling getting >24 hours protection with a KHI might still not be possible.
This means that testing the KHI each time is a necessity because when it comes down to it, all oil and gas systems are in some way unique. Consequently you very rarely rely on past tests to confirm 100% whether the KHI will work or not. You can get an idea of whether it is likely to work but you cannot know for sure until you test it using the right fluids and test conditions. An added complication of KHI testing is the stochastic nature of hydrate formation and KHI performance results in having to perform multiple runs. In early BP testing days we would test the KHI 10x times at each condition and only if it passed 8 out of those 10 times did the KHI get approved for use. Over time as our experience grew we were able to reduce the number of KHI tests run but you do need to test it multiple times to make sure the performance is at the level it needs to be.
So after performing thousands of hydrate tests, you understand what KHI’s can and cannot do and add on top of that the results from the real life field experience, you get a “feeling” for the likelihood of success depending on subcooling and system residence times inside the hydrate region. The performance range obtained from this work is represented below.
As for the above, it is worth mentioning that for years I would be in meetings where KHI’s were being discussed, and I would have project design engineers tell me that KHI’s couldn’t be used because they would only give protection for 48 hours. This was irrespective of the subcooling in the system.
Just to make it clear, this is wrong and I believe it came about because of the original KHI testing that was done for ETAP described a pass as preventing hydrate formation for a minimum of 48 hours. This scrap of information somehow over time got distorted as it was passed through the industry and morphed into all KHI’s will only ever work for no more than 48 hours. Go figure!
Now for the Chemistry bit !
Having provided some information on how KHI’s work and what their field limitations are. We can now provide some information on the chemical structures of the first KHI polymers. Knowing what chemical structures work helps during the chemical development process as you start to understand how you can improve their performance by tweeking the molecule and/or by adding synergists to increase inhibition efficiencies.
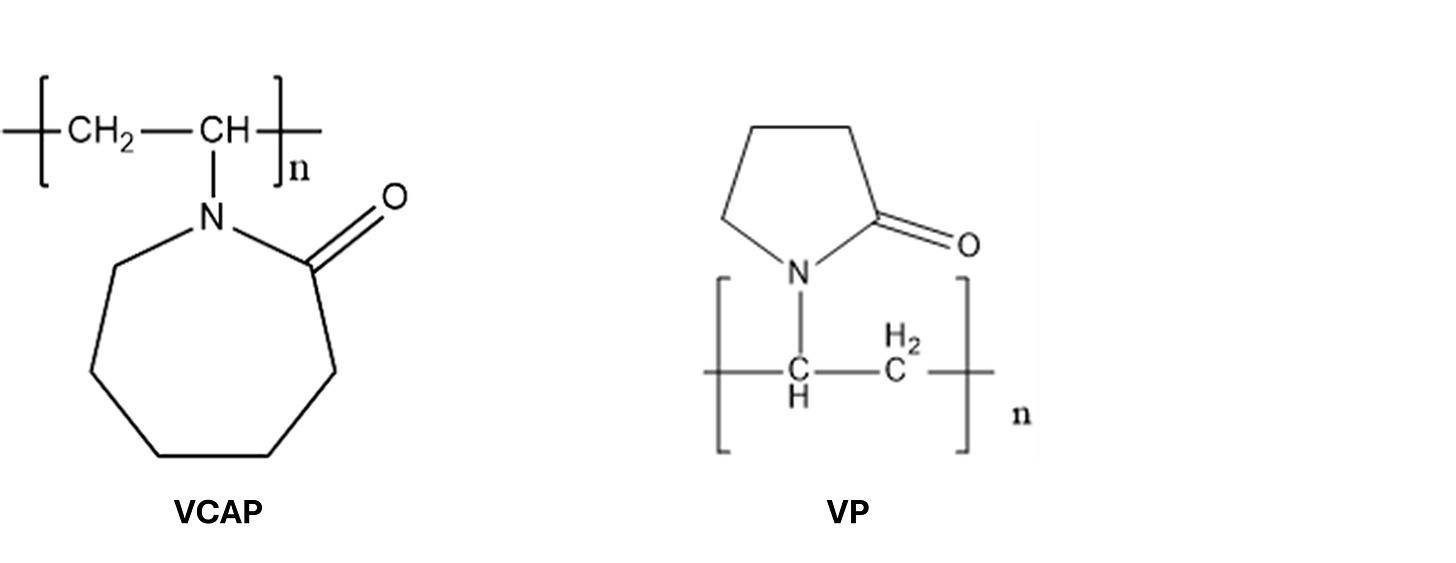
As part of their research BP identified a commercially available terpolymer with the two main monomer units being VinylCaproloactam (VCAP) and VinylPyrolidone (VP).
These monomer units were identified during the research process as having an affinity with the hydrate structure. The theory was that the amide group on the monomer units allowed the cyclic ring to fit inside / on the hydrate structure via hydrogen bonding thus preventing further growth of the hydrate crystal. For most KHI polymers it is all about whether they have the required amide group functionality.
A lot of different polymers were tested and the one that finally got commercialized was a terpolymer from a company called ISP. This was an existing commercial polymer originally used as a constituent in hair product to add a bit of stiffness to your hair ala Rikki Lake in the eponymously titled “Hairspray”. How is that for a bit of “lateral thinking”.
As well as the terpolymer, BP added an extra bit of special sauce that will remain nameless that acted as a synergist to improve the performance of the polymer itself. This was the product successfully tested in the Ravenspurn to Cleeton flowline and on numerous occasions in the lab.
Commercialization of KHI technology
So in 1996 with that as a great starting point, TROS were tasked by BP to commercialize the formulated product blend that included the commercial terpolymer for use in their Hyde/West Sole producing gas fields. This KHI application would be the first continuous offshore application of a KHI in the world. To the best of my knowledge all previous successful applications of a KHI were short term field trials.
The newly developed KHI was replacing MEG, a thermodynamic hydrate inhibitor that worked as anti-freeze type product. Not too dissimilar to what you would put in your car.
To save money the MEG was reclaimed, otherwise the volume of MEG needed was not commercially viable. The problem here was that the MEG regeneration system that BP operated onshore to recover and reuse the MEG had become extremely expensive to maintain due to salt contamination of the Rich MEG caused by formation water breakthrough at the Hyde wells.
At the same time BP also asked TROS to develop a KHI product based on the same technology used in the Hyde/West Sole KHI product for use in their ETAP development which was in the design stage at that time. This application was another first as it was going to be the first major offshore development that was designed to use a KHI from Day 1 of oil and gas production.
As part of this process TROS entered into a joint research project with BP and ISP to develop further the polymer and synergist technologies used in the existing KHI products. This helped move forward the technology by identifying new ways of improving the performance of the polymers. These included:
1. The solvent the polymer was manufactured in.
The original polymers were all synthesised in low flash point alcohol solvents but the team found that changing this to a glycol mutual solvent with a high flashpoint improved the performance significantly.
2. The molecular weight of the KHI polymer
The molecular weight of the polymers in the original KHI product were >100,000 but we saw that lower molecular weights as low as 2,000 to 5,000 had better performance and if you mixed polymers of different molecular weights performance improved further.
As all this interesting research was going on, the KHI being injected at Hyde / West Sole hit a bit of a stumbling block. This came about because the temperature at the KHI injection point was around 50oC and the produced water had a salinity >200,000 mg/l. Those two things combined led to the “cloud point” of the polymer being exceeded and the polymer dropping out as a sticky mass preventing it from working. The sticky mass that would have occurred would not have been too dissimilar to the picture below.
It was only after weeks of injecting the KHI that this problem was identified because a gradual increase in pressure drop was seen across the gas export line from the Hyde and West Sole Platforms due to hydrate formation. This obviously did not go down well but because of all the excellent research that had been done with the joint TROS/BP/ISP research project, a new polymer was quickly found to replace the existing polymer with the cloud point problem. The really impressive part was that this new polymer was then manufactured and supplied offshore so quickly no interruption to the gas supply occurred. Don’t get me wrong it did get a bit hairy, but it all turned out well in the end as it led to not only a more compatible product, it was also cheaper. In fact the BP team working on this, including myself got a BP Chairman’s Technical Award for our contributions to this world’s first KHI application, which I still proudly have to this day.
It is also worth noting that the polymer cloud point issue also impacted the ETAP development as the product selected for use there was the same KHI originally injected at Hyde / West Sole and was no longer suitable for the same reason it failed on Hyde / West Sole. What made it even more difficult was that the ETAP project needed a new product within 6 months and trying to identify a new product in that timeframe was stressful to say the least. But with a bit of hard work and a growing understanding of how the KHI technology worked. A new product was identified and supplied in the required timeframe.
All this stress did not go to waste. It helped us understand the importance of temperature and salinity to the polymer’s stability at the point of injection, which lead to a new injection compatibility test being designed to make sure the KHI when injected would stay in solution at the point of injection.
KHI uptake throughout the Industry
With the success of the BP KHI applications other operators came calling and it allowed for new field trials to be performed at onshore gas fields in the UK and France and offshore gas fields in the UK and Netherlands. For some of these field trials there were some steep learning curves.
The main ones were with Operators in the South of France and offshore the UK. The French field trial was interesting from the point of view we did all the Hydrate tests showing that it would inhibit hydrates, the injection point stability tests to make sure it didn’t come out of solution and even did some hand shaking emulsion bottle tests to show that the chemical wouldn’t form an emulsion but when the field trial came and we took a sample from the separator we got something that looked like the picture a very milky coffee.
This was definitely not expected and obviously the response from the Operator we were working with was quite rightly a strong one to put it politely.
So when I got back from the field trial, we went over everything that had been done again and still got the same results but the penny finally dropped when we tried to replicate the shearing that we thought had occurred when the gas and liquid went through a choke valve. To do this we had to use a high speed Ultraturrax mixer and as soon as we did that we got our milky coffee. So from that point on we dumped the hand shaking emulsion tests and replaced them with emulsion tests using an Ultraturrax mixer to make sure we didn’t make the same mistake twice.
As for the other KHI field mishap. I touched upon that in my substack post looking at “Lessons Learned: 30 years of MEG Reclamation Design”.
The problem there was that the KHI product was going into an existing MEG regeneration unit which operates up to 160oC. I did do some checks to make sure the KHI in pure MEG at those temperatures did not precipitate out but the problem was that I wasn’t told that there would also be up to 80,000 mg/l of salt in the MEG. Unfortunately for me the salt was included in the lab test so as soon as the KHI saw the salt MEG at the temperatures in the MEG regeneration unit you got the polymer gunk that resembled the previous picture of gunked out polymer. Again you wouldn’t have wanted to be in the room for that one.
Nevertheless, you have got to learn from your mistakes, which we did and we continued to develop new KHI products at TROS. One of our main partners in this was Total. We helped them design a new subsea oil tieback that would use KHI as their main hydrate inhibitor, a new UK subsea gas tieback and a KHI for use in a large offshore gas field in the Middle East (See SPE 8751 South Pars Phases 2 and 3: Kinetic Hydrate Inhibitor (KHI) Experience Applied Field Start-up) as a back-up solution to MEG.
The BG group also designed their Rosetta field to use KHI from Day 1 of operations due to TROS/Clariant’s success at developing KHI. Other noticeable successes during my time at TROS/Clariant where KHI applications by Wintershall and Clyde Petroleum in offshore gas fields in the Netherlands.
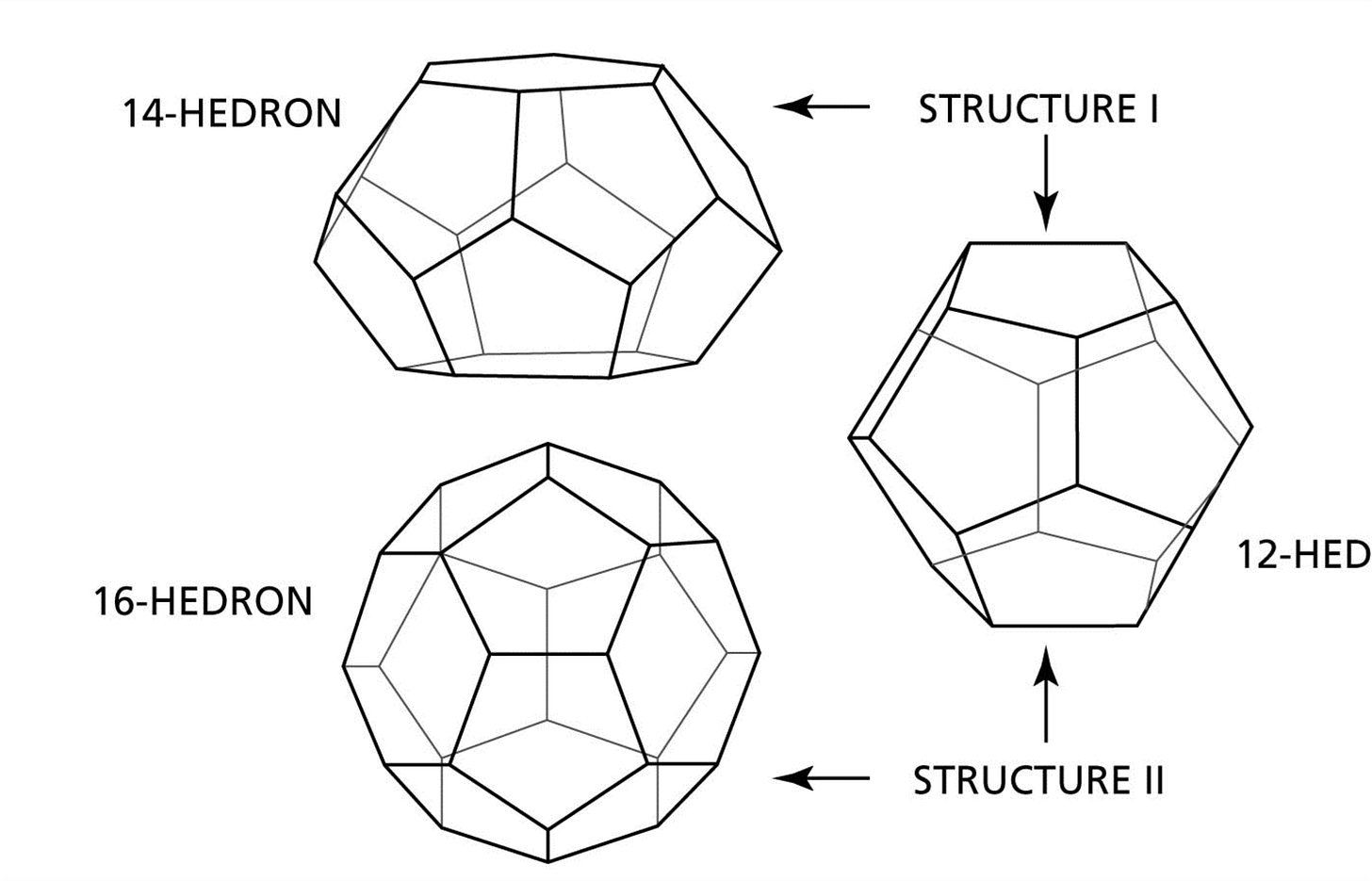
The development of the KHI for Total’s UK subsea gas tieback was an interesting one as it helped us identify for the first the difference in KHI performance depending on what Structure of hydrate was forming.
For those that don’t know there are two main types of hydrates that can form Structure I and Structure II. Before the hydrate nerds pipe up I know there is a third theoretical structure called Structure H but that has not really been shown to cause a problem in the field.
Structure II hydrates are the most common type of hydrates that form as they can fit the larger small alkane molecules like propane and butane in their structure that are found in most natural gas compositions. Whereas Structure I struggle to fit anything bigger than methane into the hydrate crystal structure so this is an issue for gases consisting of mainly methane and other Structure I forming gases. This type of gas composition is not very common but they do occur probably more than you realise.
For the UK subsea gas field, the gas composition was >98% Methane with the remainder mainly being CO2 and N2 so it was forming a Structure I hydrate. At the time we didn’t realize this would be a problem as the subcooling was well within the capabilities of KHI’s already being used at 8-10oC subcooling. But when we started to do our testing we couldn’t get anything to work and after a while we were just scratching our head as we didn’t understand why that should be the case. It then finally dawned us that the products we were testing had all been designed to work on Structure II hydrates so the active parts of the polymers were sized to fit the bigger cavities available in Structure II hydrates. So as soon as we realized that and focused on products better suited to the smaller cavities present in Structure I hydrates, the performance of the KHI tested started to improve.
With more and more KHI’s being used in the field we started working with more polymer suppliers and one of the things we learned from that was not all polymer batches delivered had the same performance due to the vagaries of the polymer manufacturing process. This led to the requirement to perform QA/QC test on each new polymer manufacturing batch received to make sure the performance of the KHI in the field was as expected.
With all the testing we were doing, we also found the KHI performance was sensitive to the presence of other production chemicals. This was very noticeable when we had to develop combined KHI/Corrosion Inhibitor products. So when testing KHI’s always think about what other chemicals may be being injected.
New Doors Opening
While at TROS/Clariant I was lucky to come into contact with many of the major Operator’s Hydrate SME’s, got to present to Dendy Sloan (The godfather of Hydrates) on a KHI application in an American Operator’s offshore gas field in the UK Southern North Sea and presented at conferences on the use of KHI’s.
This set me up very nicely for my next career move because one of these connections got me a job with Shell as a Hydrate Specialist in their Flow Assurance Team based in Amsterdam. It was probably one of the best workplaces I had the pleasure of working in and there was the added novelty of getting to go to work via a ferry every day. Good times!
Most of the site no longer belongs to Shell but when I was there all you can see in the picture was Shell’s site.
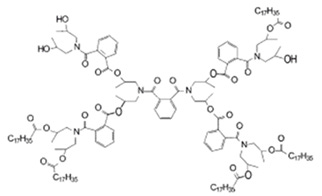
The focus at Shell was much more on hydrate management design but there was still time to have some KHI side projects. My Shell team worked with DSM, a major Dutch chemical manufacturer and Baker Hughes to commercialize a new KHI product based on a dendritic polymer.
Example of a Dendritic Polymer
This was quite novel as all the polymers that I had worked on previously were your more conventional linear chain polymers. With the experience gained developing KHI for TROS I was able to help Baker Hughes get a competitive KHI formulation that not only had good hydrate inhibition performance at a cost effective dose rate. It had good brine compatibility as well.
The main KHI project when I was at Shell involved confirming the feasibility of using a KHI in a major offshore Shell gas asset in the Middle East as an alternative to a high CAPEX MEG reclamation unit.
After I left Shell I thought I was done with KHI’s but while at Woodside Energy I was able to work with CSIRO on confirming whether it was feasible to mix a KHI with MEG that would go through a MEG regeneration package. The outcome of that study confirmed that it was possible, but I left Woodside before I could do anything with the findings.
I have now come full circle with Pontem Analytics where we have been asked on a few occasions to help out with determining the feasibility of KHI’s and what I have found out is that all I learnt all those years ago at TROS is still 100% relevant today.
Finally, as a little taster of what we could do for you. I will leave you with a good high level description of the sort of process you would need to follow if you were wanting to get a KHI from the lab to the field.
So, if there is anybody out there that wants to learn more about KHI’s feel free to contact Pontem Analytics we are ready to help.