Lessons Learned: 30 years of MEG Reclamation Design
This MEG doesn't have teeth!
Here at Pontem Analytics, we are lucky to work on some of the biggest gas projects happening in the energy industry just now. These are located all around the world, in places like the Caribbean, West Africa, East Mediterranean and Australia. These may be scattered around the globe but they all have one thing in common: the use of Mono Ethylene Glycol (MEG) injection and recovery as their primary hydrate mitigation strategy. MEG if you didn’t already know it is not only used as a de-icer in cars (antifreeze), but is also a very effective hydrate inhibitor.
When mixed with water produced from the reservoir, MEG helps prevents hydrates from forming. But, the secret to a cost-effective strategy is the MEG is operated in a closed-looped system, where the chemical is purified and recirculated. Without recovering the MEG from the water, the MEG volumes used would be massive costing $10’s millions if not $100’s millions / year in OPEX. To minimize these costs, distillation processes have been developed to allow the MEG to be separated from the water and recovered. These are what we call MEG recovery units (MRUs).
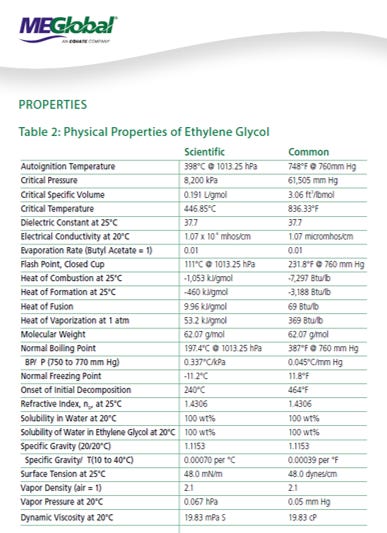
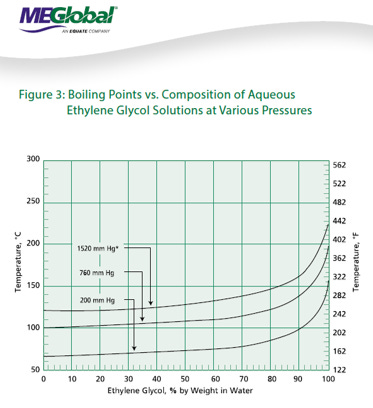
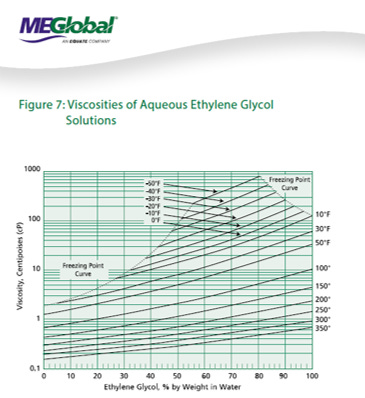
We will walk through some of the common pitfalls with MEG system design, as well as include some very useful references on MEG properties (courtesy of MEGlobal).
20th Century MRUs
I first came across MRU’s nearly 30 years ago via a circuitous route. Initially, I was developing and applying some of the first commercial Kinetic Hydrate Inhibitors (KHI) and as part of this role, I used to get phone calls from operators asking whether a KHI would work for them. As it turned out, a significant number of the operators that contacted me had problems with their MEG regeneration systems due to fouling caused by formation water production. These were significant enough that they were looking at using KHI as a way to replace MEG. In fact KHI in a number of these occasions successfully replaced the use of MEG.
Nevertheless, it was not all plain sailing for KHI’s. One eventful KHI field trial had the production stream containing a KHI feeding into an existing MRU containing salty MEG. Even though we had tested the KHI to make sure it would be compatible with pure MEG at the high temperatures seen in the MRU we had not taken into account the MEG contained up to 80,000 mg/l of salt and unfortunately that made all the difference. The additional salt caused the KHI to drop out of solution as a sticky, gooey mess that started to block everything and thus threatening to shut down the field. That was bad enough but this field in particular supplied at the time nearly 20% of all gas to the UK. Trust me being in those meetings trying to explain what had gone wrong was not pleasant.
By I digress, this post is about MEG not KHIs. So back to MEG, what I found out about MEG systems then was that the first-generation MRU mainly consisted of reboilers designed to boil the water off from the MEG. These were usually fine when only condensed water was being produced with the gas as there was very little salt present. But even then, there were examples of where fouling became a problem due to the dissolved iron from the corrosion of the carbon steel flowlines forming scales such as iron carbonate and iron oxide (magnetite).
Things became really difficult to manage when formation water was produced along with the gas and condensed water. When that happened due to the nature of the MEG reboiler the additional salt that was produced just accumulated in the MEG and once it reached anywhere above 50,000 to 80,000 mg/l of total salt, it really started to foul the MEG reboiler because at the temperatures in the reboiler ~160oC halite (solid NaCl) crystals started to drop out of solution. Once this started the MRU became virtually inoperable and the only solution was to replace the salty MEG with new MEG. This not only cost vast amounts of money but logistically was really difficult due to the large volume of salty MEG that had to be removed and then safely disposed of.
At that time some UK operators were trying out salt removal methods. One operator had one of the first vacuum reclamation systems used in the industry and another had an ion exchange membrane that removed the unwanted salts. However, even these systems had their problems and were difficult and costly to operate.
MRUs Today
Move forward nearly 30 years and the situation is now much improved due to the refinement of the vacuum processes and the development of pre-treatment systems to remove the “bad actor” salts before they become a problem.
The vacuum reclamation systems allows for the removal of the soluble monovalent salts such as NaCl and KCl from the processed MEG so removing the salt accumulation problem and the pre-treatment system removes the divalent ions such as Ca2+, Fe2+, Mg2+, Ba2+ and Sr2+ before they reach the Reboiler. If they remain in the Rich MEG even at low level concentrations, they will foul the MEG reboiler where temperatures are in excess of 160oC. They do this by forming insoluble scales such as Fe2CO3, Fe2O3 / Fe3O4, CaCO3, etc.
Fouled MEG Reboiler
MEG pre-treatment systems work by intentionally precipitating out the divalent ions from the Rich MEG in a controlled manner so that they do not reach the MEG Reboiler. It does this by heating up the Rich MEG using a solids friendly heat exchanger (such as a spiral heat exchanger) to a temperature (80-90oC) that is high enough to start precipitating out the divalent ions when an alkali solution such as sodium hydroxide or sodium or potassium carbonate is added to the Rich MEG to raise the overall pH of the systems.
Spiral Heat Exchanger
By increasing the pH of the system, the solubility of the divalent ions reduces, and they start to come out of solution as solids. Once that happens, the intentionally precipitated solids are removed from the Rich MEG by filtration or centrifuging. After this point the Rich MEG is now devoid of the scaling ions that caused the fouling of the MEG reboiler.
Key points to note are:
1. Sodium hydroxide is usually preferred as you need to add less of this chemical as it is a stronger alkali, but when using this chemical, you need enough dissolved CO2 from the produced gas in the Rich MEG to ensure that there is enough carbonate created to react with the divalent ions. If that is not the case that is when sodium or potassium carbonate is used instead.
2. Once the pH is raised in the Rich MEG it is important to make sure that does not adversely increase the pH of the Lean MEG after processing. High pH Lean MEG is not good because when injected at the same point the formation water is being produced, it can cause inorganic scaling to occur at that point in the system. If that occurs you may have to reduce the Lean MEG pH by adding an acid to the Lean MEG. This is just another operational step and cost you would prefer to avoid.
3. It is important that you have sufficient sparing of the heat exchanger that is part of the pretreatment system as they eventually become fouled themselves. Most of these systems have effective clean in place (CIP) capabilities that allow for quick removal of the carbonate scales deposited by circulation of a suitable acid scale dissolver.
Lean MEG Concentration
As we treat the MEG to clean out the solids and reboil (concentrate) the remaining MEG to an acceptable purity, we can then go back out to the field with our “lean MEG”. Did you know that antifreeze can freeze? Yes, this is why we manage the water content of the lean MEG, typically in the 80 - 90wt% range, which is a good balance between freezing concerns, dosage optimization, and viscosity concerns.
In the post above, we tried to give a very high-level evolution from a personal perspective of how MEG reclamation systems have changed over the years and some of the reasons for those changes. We also tried to drop some hints on where the common pitfalls were. Such as:
Even when there is no formation water production, if you have only a MEG reboiler and/or no pre-treatment system, you will still get fouling because of the dissolved Fe2+ from the corrosion process.
With a MEG reboiler, you can usually only manage up to 50K-8K mg/l salt before halite starts dropping out
Our experience has been that the best heat exchanger to use for pre-treatment systems is typically a spiral heat exchanger
Control of MEG pH is a critical step to bring balance to all of the associated chemistry issues, some of which are competing with each other
Sparing is good
I think the above shows the Good, the Bad and the Ugly parts of using MEG. It is not all plain sailing, but once you know the do’s and don’ts, it can be a very effective method for preventing hydrates. With that in mind, let Pontem Analytics guide you through the process of designing and operating a highly-efficient MRU.
Tommy’s Note: Speaking of freezing / hydrate prevention…if you happen to be in Norway visiting the leading MRU research institute (IFE, Institute for Energy Technology), make sure to take the short drive from Kjeller to Sweden for some skiing. You know, just to test the antifreeze min temp in your car is safe down to -20C (yep, 40/60 glycol mix worked just fine)…
Please reach out to info@pontemanalytics.com to discuss how we can help solve your business’s most important problems!