Hydrate Quiz
Test your Subject Matter Expertise
<UPDATE: Answer key now added in bold>
We are all about the science (and data) at Pontem Analytics but we also like to have some fun, so we decided to combine the two. With that in mind we have put together a quick little Hydrate Quiz. To get all you budding and existing Flow Assurance Engineers thinking. And, if you have been reading our hydrate posts, this should be a breeze…
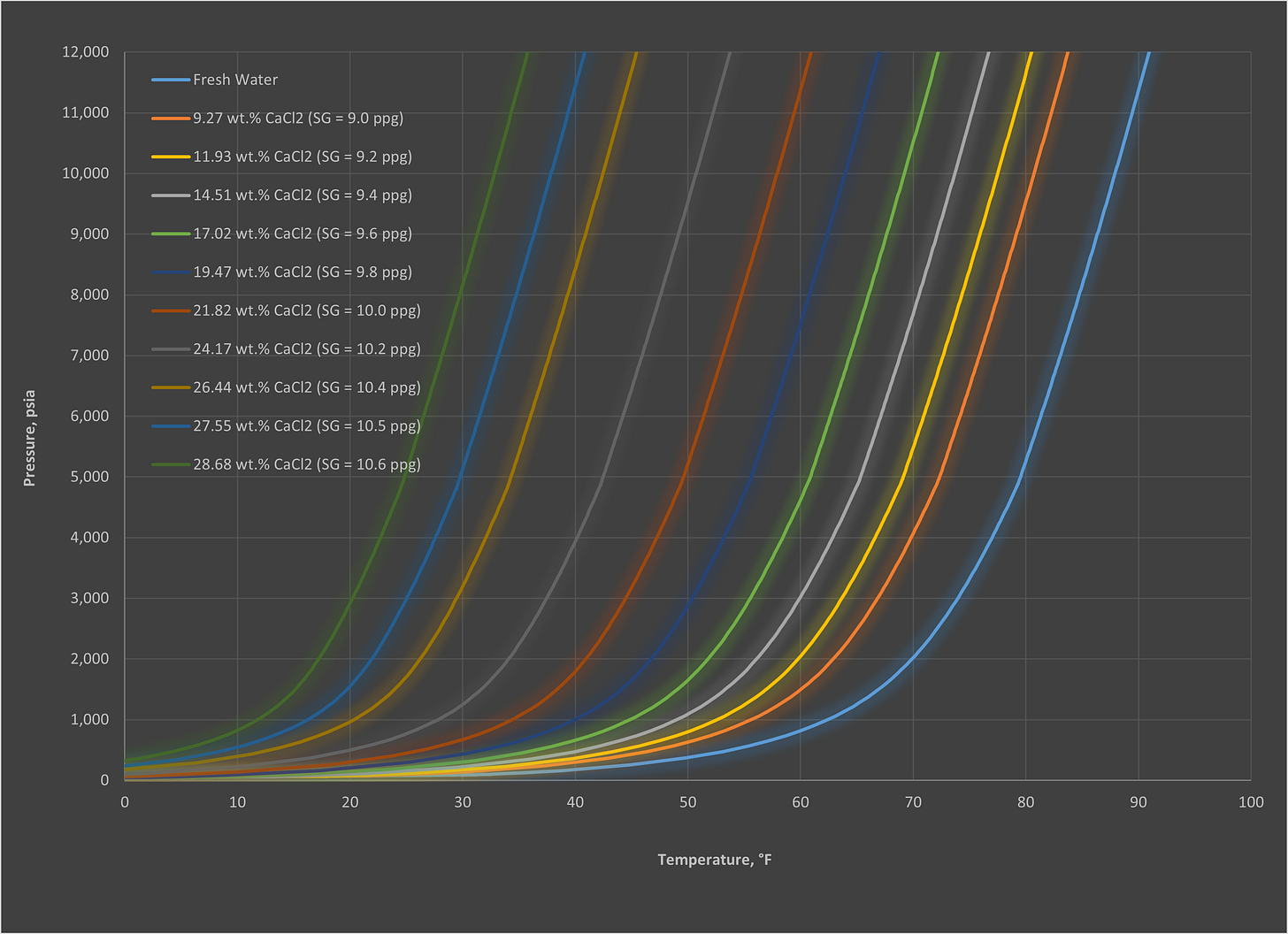
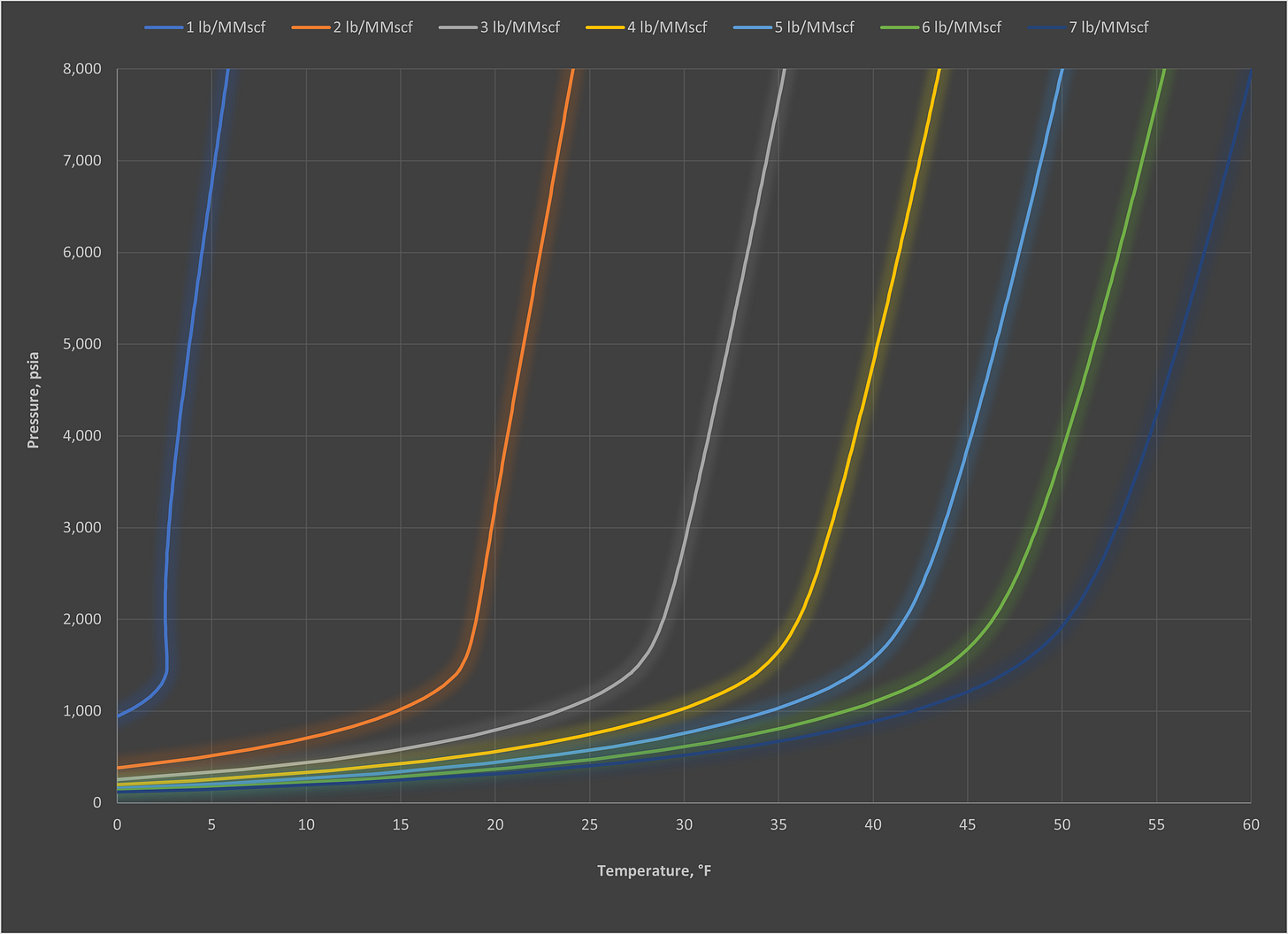
As with most things there are a lot of misconceptions around hydrates and the actions you can and cannot do to manage them. Therefore, we thought we would share some questions to help put these to bed. We have also included some of the most commonly-requested hydrate curves, so use them for reference as you see fit (all generated via Multiflash, CPA EoS).
There is no prize here other than bragging rights over your friends when you get more right than they do, which should be incentive enough. Or, if you want the answer key, message us your answers and we will (a) publicly announce our first 20/20 perfect sheet and (b) provide you the correct responses.
Side note - guessing “C” throughout university served some of us well, so maybe go with that when in doubt
Kinetic Hydrate Inhibitors cannot delay hydrates longer than 10 hours
Yes, 10hrs is a walk-over
No, 10hrs is too long
Depends on the subcooling
Only in black oil lines
For a system of given composition the hydrate formation temperature is sharply defined if also
The pressure is known
The pressure and the temperature are known
The pressure, the temperature and the cooling rate are known
The hydrate formation temperature is not sharply defined, only the hydrate dissociation temperature is
Methanol can always be used to melt a hydrate plug, but MEG can never be used to melt a hydrate plug
Always true
Always untrue
Depends on the location of the plug
Depends on the pressure, the temperature, the location and the permeability of the plug
When there is a hydrate plug in a vertical pipe and you can only inject chemical from above what is the most efficient chemical to inject to remove the hydrate plug
Methanol
MEG
LDHI
None of the above
A gas which is predominantly methane (>98%) is in contact with water at a temperature of 5°C and is capable of forming thermodynamically stable structure I and structure II hydrates. Which of the following statements is correct regarding this gas?
Structure I hydrates are stable at higher pressures than structure II hydrates
Structure I hydrates are stable at lower pressures than structure II hydrates
Both structure I and II hydrates are stable inside the hydrate region
Such a system will neither form structure I or structure II hydrates, structure H hydrates are formed instead
An Arctic pipeline that operated outside the hydrate region without inhibitor is blocked by hydrates after an unplanned shut-in. The temperature in the pipeline has now dropped below zero. What happens after the pipeline is depressurized?
The hydrate plug dissociates and pressure communication is established
The hydrate plug converts into an ice plug
The hydrate plug remains unchanged
None of the above
An operator can chose to inject 100 tonnes/day of methanol or MEG to prevent hydrates in a gas/condensate line. Which inhibitor will result in the lower dissociation temperature?
Methanol
MEG
Effectiveness of the chemical depends on the water-gas ratio
There is no difference
A live oil line that operates above the bubble point but inside the hydrate region (the line contains only bulk oil and bulk water phase, no free gas phase) can never become blocked by hydrates. Is this
Generally untrue
Generally true
Only if the water cut is less than approx. 40%
Only if the water cut is higher than approx. 40%
Two process engineers want to calculate the temperature downstream of a Joule Thomson choke through which gas expands from 150 to 30 bar. Process engineer A assumes isenthalpic expansion. Process engineer B assumes isentropic expansion. Process Engineer B finds temperatures that are tens of degrees lower than operator A. This is because
Heat is transferred to the environment during isentropic expansion
Heat is taken up from the environment during isenthalpic expansion
Internal energy of the gas is converted into kinetic energy of the gas during isentropic expansion
Isentropic expansion results in lower pressures
A vessel having a volume of 100L contains 10L of water and 90L of methane at a pressure of 100 bar. 10L of decane are added whilst the volume of the vessel is expanded to keep the pressure constant at 100 bar. The hydrate dissociation temperature of the water/methane/decane system is
Practically the same as for the water/methane system
A few degrees lower than the water/methane system
A few degrees higher than the water/methane system
The hydrate dissociation temperature does not exist after decane is added because hydrates cannot form anymore
A super major has a deepwater gas field they want to tie back to an LNG plant. When producing it will continuously operate inside the hydrate region with a subcooling >15oC and virtually no hydrocarbon condensate. With it operating inside the hydrate region it will need continuous hydrate inhibitor injection. What hydrate inhibitor will they choose?
Methanol
KHI-LDHI
MEG
AA-LDHI
If you had 1000 bbls of water to inhibit and you were able to mix the water directly with your chosen thermodynamic hydrate inhibitor at the same percentage dilution which chemical would give you the most hydrate inhibition.
10% DEG
10% Methanol
10% MEG
10% TEG
What is the best model to use when trying to calculate the dose rates of thermodynamic inhibitors such as Methanol, MEG, DEG, TEG and salt?
Peng Robinson (PRA)
Redlich-Kwong-Soave (RKSA)
Peng Robinson 1978 (PR78A)
Cubic Plus Association (CPA)
When a gas composition consists of 99% Methane and 1% CO2 what type of hydrates are formed?
Type I
Type II
Type H
A mixture of all three Hydrate types
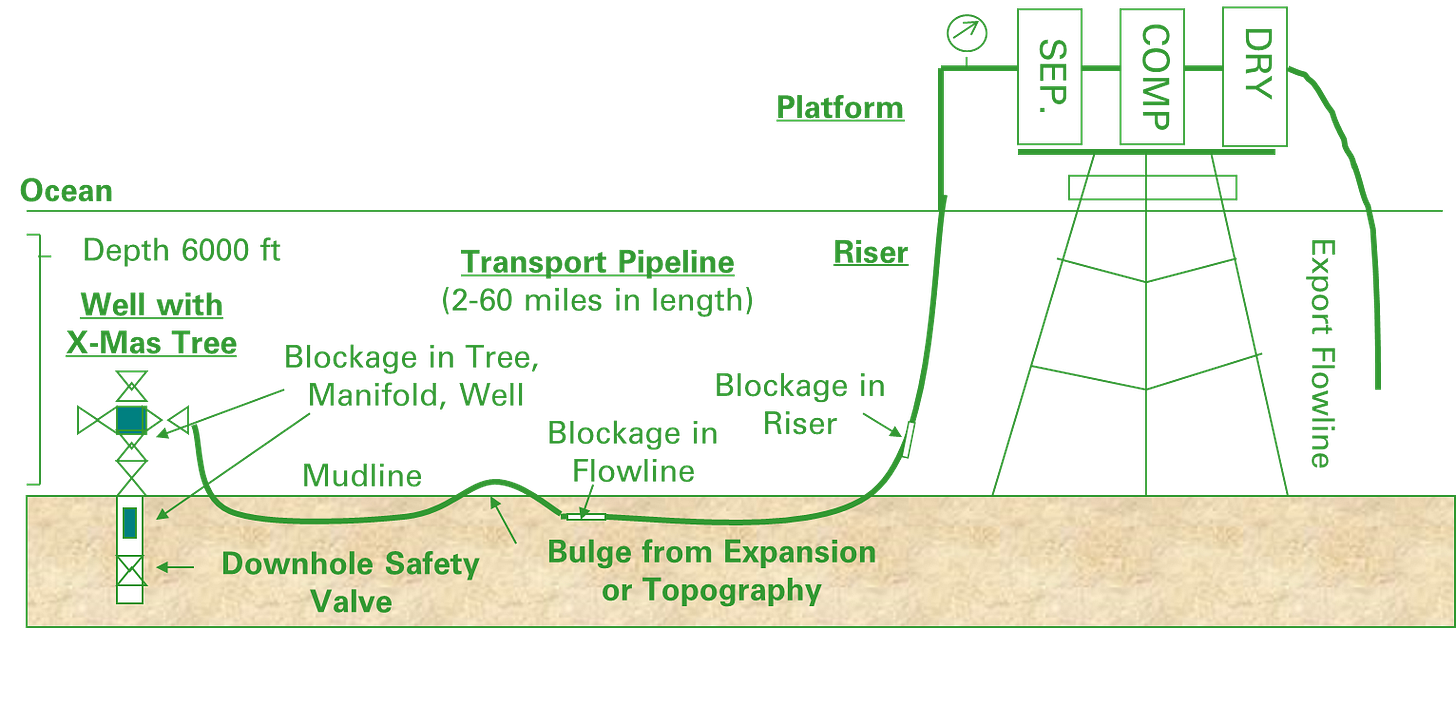
Where is the most common location in the well for the SCSSV?
As close to the wellhead as possible
Nice and deep so it is lovely and hot and well outside the hydrate region
The geothermal temperature which just sits outside the hydrate region at the wells SITHP
Doesn’t matter it can go whether you want to put it
For a 24” gas line that as a 1% water fraction with minimal liquid hold-up operates continuously inside the hydrate region. It normally injects MEG to prevent hydrates from forming but there is a problem with the MEG pumps and MEG injection has stopped. How quickly will the line plug?
Minutes
Hours
Days
Weeks to Months
What does some research say is more likely to cause a hydrate blockage?
Dosing no Methanol
Underdosing Methanol
There are two 8” subsea lines side by side sitting on the seabed at exactly the same 100m water depth and temperature. One is uninsulated and the other has insulation with a U-value of 4 W/m2K. Somehow, they have both managed to get blocked by hydrates at exactly the same point. Both lines are depressurized from both ends exactly at the same time and at the same rate. Which line will have its hydrate blockage removed first:
Uninsulated line
Insulated Line
In the last 30 years more and more systems that use MEG for hydrate inhibition recover the MEG using a Vacuum reclaimer. What is the primary function of the Vacuum reclaimer:
To remove oxygen
To separate the soluble salts such NaCl and KCl from the MEG
To remove any hydrocarbon liquid from the MEG
None of the above
In 1934, this American chemist showed that hydrates were causing blockages in natural gas transmission lines. His formulas (shown above) were the early prediction tools to estimate wt% of thermodynamic inhibitors such as MeOH and MEG to inhibit a known amount of subcooling.
Hadjicharalambous
Hamsandwich
Hackenschmidt
Hammerschmidt

Hopefully the questions won’t wrack your brains too much. What we do hope though is that after you have done the quiz you will sleep better knowing you have your hydrate problems under control and don’t worry if you don’t, we at Pontem Analytics are here to help, hydrates are our thing.
Finally, there is only one rule, the Quizmaster’s decision is final and you will have to read our next post to get the answers.
Please reach out to info@pontemanalytics.com to discuss how we can help solve your business’s most important problems!






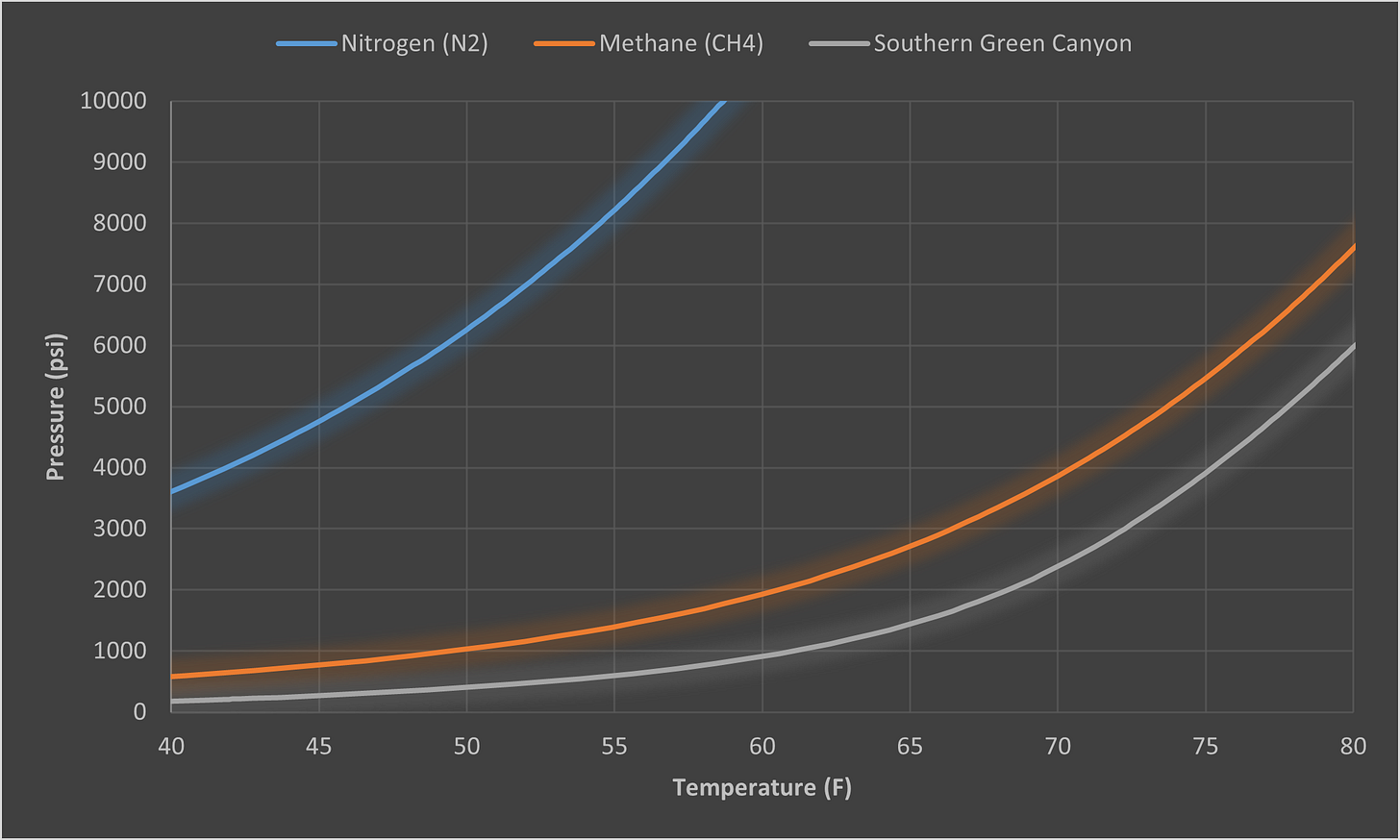

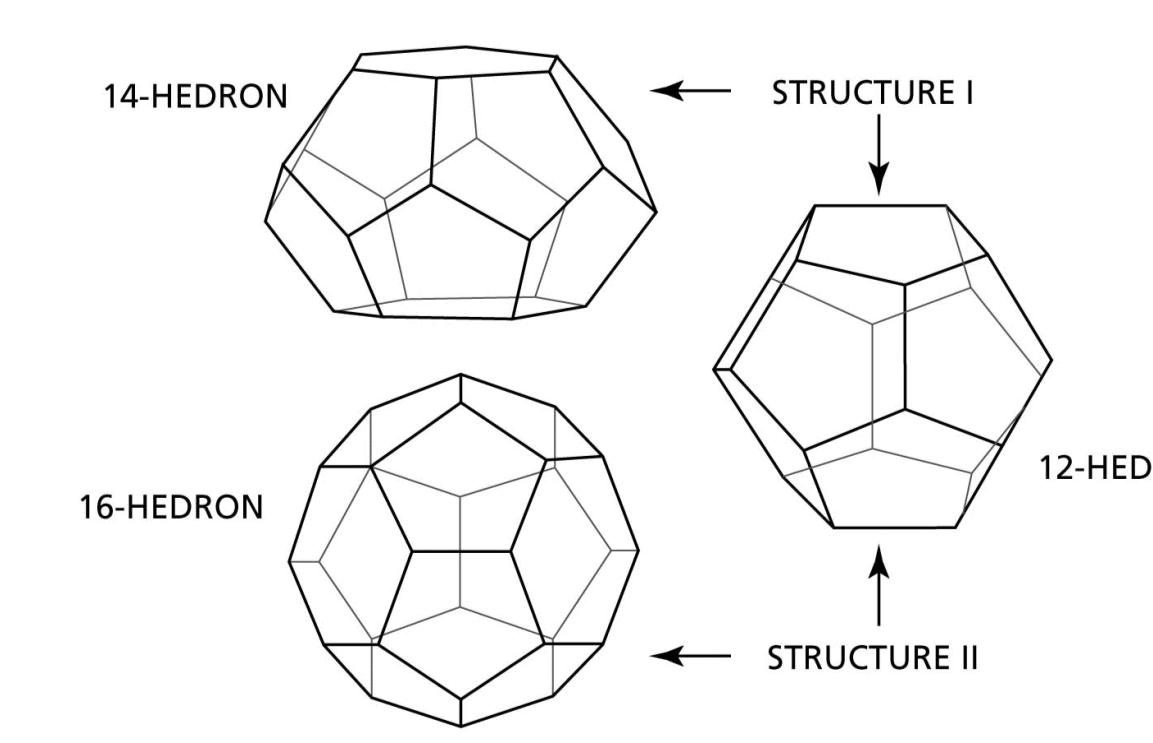


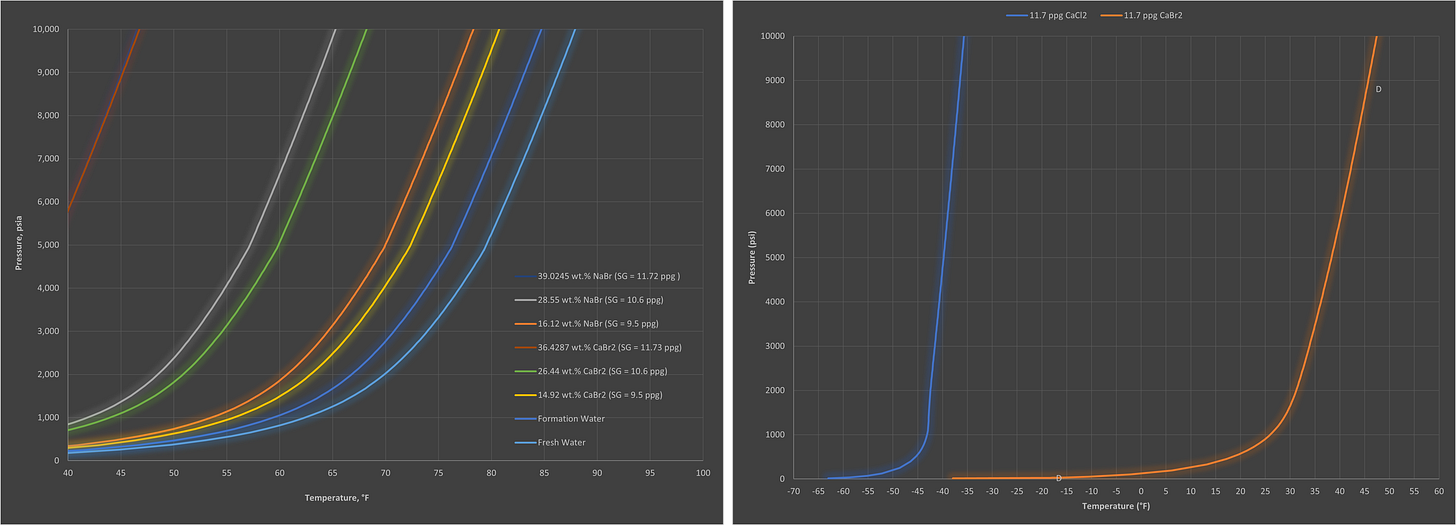

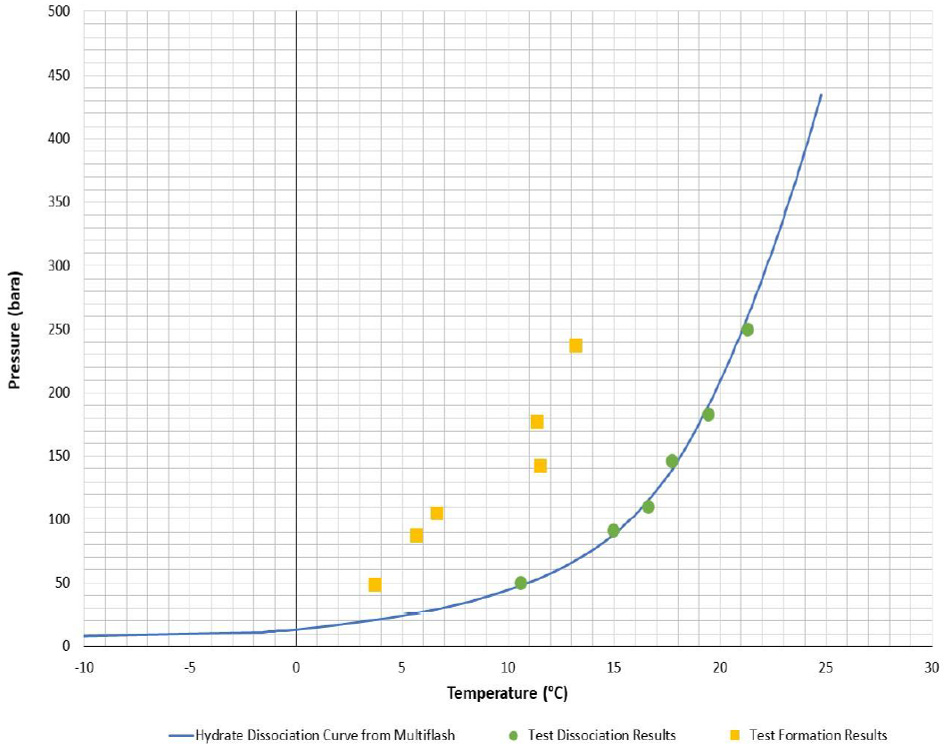





Excellent post! Thank you.
Here are my answers: 😊
1) C
2) A
3) D
4) A
5) B
6) A
7) B
8) A
9) C
10) B
11) A
12) D
13) D
14) A
15) C (I think 😊 )
16) C
17) B
18) B
19) B
20) D
Cheers,
Behnam (Multiflash Product Manager)